Current status of LA/ER Cabotegravir (CAB) and Rilpivirine (RPV)
including pipeline report on novel CAB formulations
Speaker: William Spreen, Cabotegravir Medicine Development Leader, ViiV Healthcare

Progress in CAB LA for HIV PrEP
- Four regulatory approvals (US, Australia, Zimbabwe, and S Africa) and multiple submissions under active review (European Medicines Agency [EMA], Brazil, and sub-Saharan African countries).
- The WHO has issued guidelines for use.
- Indication – at-risk individuals >35kg, optional OLI, no contraindication in pregnancy or lactation, HIV testing via RNA-based test at initiation in US or per national guidelines elsewhere.
Data from HPTN-083 (MSM/TGW) and HPTN-084 (cis-women) illustrate the staying power of LA HIV prevention.
- CAB LA maintains efficacy advantage over oral TDF/FTC with consistent hazards reductions in incident HIV infection across blinded and unblinded phases – 66% risk reduction in HPTN-083 (CROI 2022) and 89% risk reduction in HPTN-084 (AIDS 2022).
- ViiV and Medicines Patent Pool signed a voluntary licensing agreement in July 2022.
- Selected generic manufacturers can supply generic CAB LA for PrEP in 90 countries.
- Multiple oral presentations at CROI 2023.
- Susan H Eshleman et al. The LEVI syndrome: characteristics of early HIV infection with cabotegravir for PrEP.
- Mark A Marzinke et al. Cabotegravir pharmacology in the background of delayed injections in HPTN 084.
- Hyman Scott et al. Cabotegravir for HIV PrEP in US Black men and transgender women who have sex with men.
- Sybil Hosek et al. CAB LA for HIV prevention in African cisgender female adolescents (HPTN 084-01).
Bi-monthly CAB + RPV LAI for HIV treatment.
- CROI 2023 presentations.
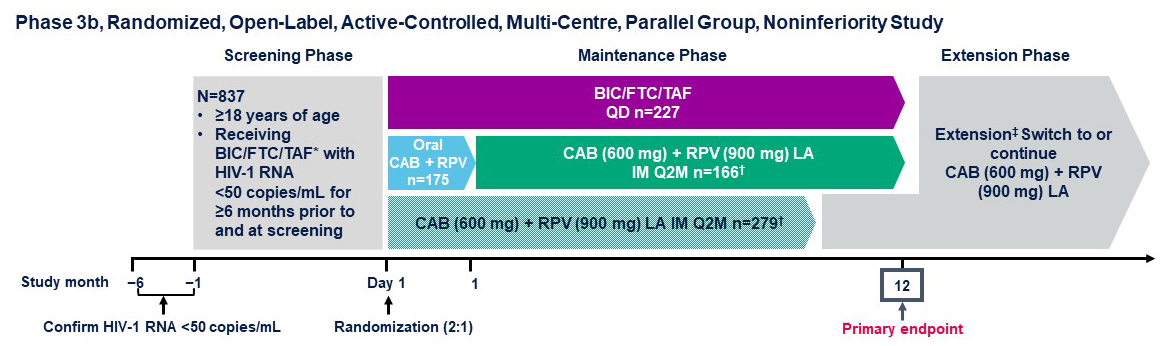
- SOLAR Study 12-month results.
- Moti N. Ramgopal et al. Randomized switch trial of CAB + RPV LA vs oral B/FTC/TAF.
- Darrell HS Tan et al. Weight and metabolic changes with cabotegravir + rilpivirine long-acting or bictegravir.
- ATLAS-2M Study. Franco Felizarta et al. Thigh Injections of Cabotegravir + Rilpivirine in Virally Supressed Adults with HIV-1.
- SOLAR Study design.
Metabolic endpoints include change (Day 1 to Month 12) in: BMI category; waist and hip circumferences; waist-to-height ratio; waist-to-hip ratio;
and proportion with insulin resistance or metabolic syndrome.
- ATLAS-2M Study – Phase 3b sub-study evaluating CAB + RPV via IM thigh injections in virologically suppressed PLWH.
New opportunities to extend dosing intervals using a novel double-concentrated CAB400 mg/mL formulation and rHuPH20-facilitated administration.
- Phase I healthy volunteer study (212482) of CAB400 (multiple doses and routes of administration) vs approved CAB200 (AIDS 2022).
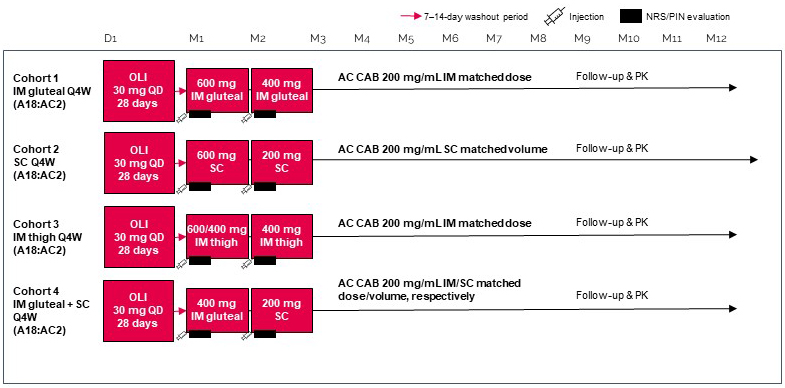
- Similar safety profile – Grade 1-2 ISRs common and short-lived.
- Unexpectedly higher CAB400 absorption rate– higher Cmax and shorter terminal half-life. Plasma concentrations within the range of CAB200 regimens, regardless of route.
- CAB400 practical for Q1M dosing, but dose/volume impractical for longer intervals.
- Studies evaluating hyaluronidase-facilitated administration to allow larger injection volumes with potential to achieve ≥Q3M dosing intervals.
- Halozyme’s recombinant hyaluronidase Ph20 enzyme (rHuPH20) temporarily expands the SC space (permeability barrier restored in 24 to 48 hours).
- ViiV-Halozyme studies are evaluating rHuPH20 administration with HIV therapeutics across multiple targets (INSTIs, NRTIs, Capsid Inhibitors, bNAbs).
- Study 218012 is ongoing – safety and PK of approved CAB200 and novel CAB400 formulations (multiple doses and routes of administration) with and without rHuPH20; no OLI required (NCT05418868).
Other LA initiatives.
- Self-administration – FLAIR sub-study of sub-cutaneous dosing of CAB + RPV LA to assess safety, tolerability, and PK in participants experienced with CAB+RPV LA via gluteal IM dosing (NCT02938520).
- Longer SC dosing intervals – CAB + RPV LA SC in healthy participants (with Janssen).
- Other LA regimens.
- ACTG 5357 Study of CAB LA + VRC07-523LS for HIV suppression (NCT03739996).
- Upcoming study of CAB LA + bNAb N6-LS (VHC3810109).
Summary
- Additional regulatory approvals and expansion of datasets for HIV PrEP with CAB LA and HIV treatment with CAB + RPV LA.
- Novel CAB formulations are in clinical trials.
- ViiV-Janssen collaboration evaluating additional LA opportunities with CAB + RPV LA regimen.
- ViiV-Halozyme collaboration with rHuPH20 may enable new 2-drug regimens with other clinical candidates.
