Physiological based Pharmacokinetic Modeling to Support
a LA HIV Drug-combination Formulation
Speaker: Simone Perazzolo, Targeted Long-Acting Antiretroviral Therapy (TLC-ART) Program,
Department of Pharmaceutics, University of Washington

“PBPK is perhaps the best tool for assessing long-acting feasibility in humans”
Validated PBPK models are a predictive tool rooted in physiology and mechanisms. How the TLC-ART platform (Class II) generates LA differs from existing slow-release products (Class I) and requires a different modeling approach.
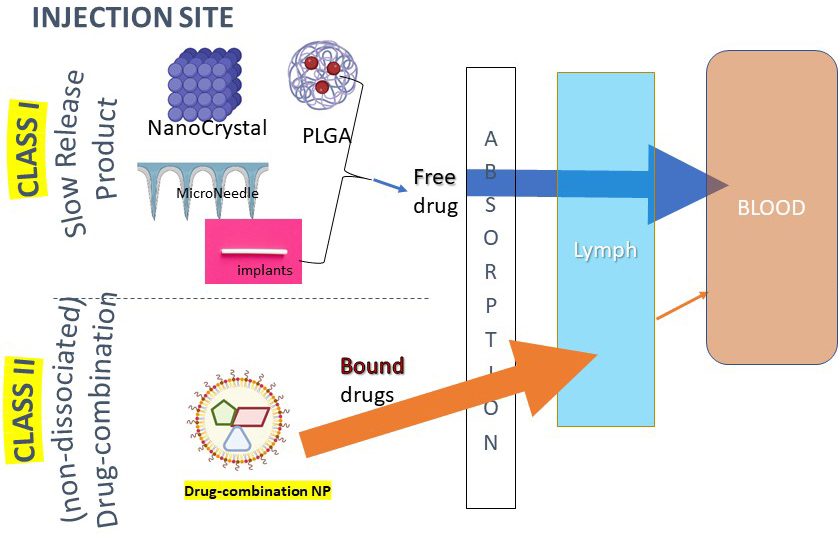
- “Lag PK profile” of Class I slow-release products (example: LA CAB).
- Nanocrystals, microneedles, and implants are engineered to slowly and continuously release free drug from the depot at the injection site.
- Free drug is released and quickly absorbed into the blood compartment (first phase).
- Requires time to achieve first peak therapeutic concentrations – delay in treatment.
- Slow release from the depot determines the LA profile (key kinetic controller). Pharmacokinetics depends on how the product is engineered.
- “No-lag PK profile” of Class II non-dissociated drug-combination products (example: LA Dolutegravir in NHPs).
- Drug-combination Nanoparticles (DcNPs, nanoparticles associated with a combination of ARVs) are formulated to remain stable. Physiochemical properties (size, shape, charge, etc.) are not suitable to stay in the local injection site or directly cross into the blood.
- Bound drug quickly absorbed into the lymph compartment – sequential spread across nodes exposes the whole lymphatic system to ARVs (first phase).
- Can achieve first peak therapeutic concentrations in 6 to 24 hours – no delay in treatment.
- Slow release from the lymphatics determines the LA profile (key kinetic controller).
Building on the LEAP/Univ of Liverpool modeling approach, we constructed and validated a PBPK model in NHPs for SC administration of TLC-ART 101 (DcNP containing LVP/RTV/TFV).
- Goals: Explain LA mechanisms in NHPs; Include elements of scaling to humans; Feedback in formulation to optimize the fix-ratio for better LA; and Evaluate feasibility in children.
- PBPK models developed for the free-ARV mixture and the DcNP formulation (two publications in Journal of Pharmaceutical Sciences 2022).
- Model 1 (free-drug mixture) – how the PBPK model needs to be adapted.
- Model 2 (DcNP formulation) – the journey of NPs from the injection site, across the lymphatics, to the blood.
- TLC-ART PBPK model validated in NHPs (plasma [PBMCs], lymphatic system [LMNCs], tissues, etc.).
- Validation using first-in-human (FIH) data would support scaling to humans and potentially children – we now have approval to proceed.
PBPK modeling of TLC-ART 101 filled mechanistic knowledge gaps of our DcNP delivery system – non-dissociated DcNPs remain bound during transit from the injection site to the lymph nodes and are retained in the nodes for enhanced lymphocyte exposure.
- Modeling at 2 weeks (one half the interval dose).
- High clearance rate at SC injection site (no depot) – fast absorption of DcNPs into the lymph nodes (70% of dose).
- Low clearance rate at lymph nodes – excess DcNP enters the blood (30% of dose), with PBMC >plasma.
- Intermediate clearance rate at blood – ARVs dissociate in the blood, and APIs are eliminated according to native clearance (renal/biliary).
- IV administration of TLC-ART 101 in NHPs informs the PBPK model.
- Useful for bioavailability considerations and to establish the in vivo association efficiency of the NPs (important to support scaling from in vitro to in vivo stability).
TLC-ART PBPK modeling can be used to “open the lymphatic system.”
- Exposes all the nodes and provides time courses.
- Example of protease inhibitor (LA CAB) time course using nodes collected from NHPs at necropsy.
Conclusions.
- PBPK modeling enabled us to fill knowledge gaps in the mechanisms of the TLC-ART delivery system (mono-clonal antibodies may have similar mechanisms).
- Published TLC-ART PBPK model supports DcNP development.
- Offers lymphocyte and lymph node resolution, including traffic of lymphocytes in and out of the node.
- Can track whether ARVs are free or bound to NPs at any timepoint.
- Clinical validation of the model will support formulation and dose selection for adults and PK scaling for children.
