Targeted Long-Acting Combination Antiretroviral Therapy (TLC-ART)
Program Updates pipeline report on novel CAB formulations
Speaker: Rodney Ho, PI, TLC-ART Program at University of Washington

Overview of the TLC-ART Program to date – from concept to translations to initiating Phase I first in-human (FIH) studies for HIV treatment.
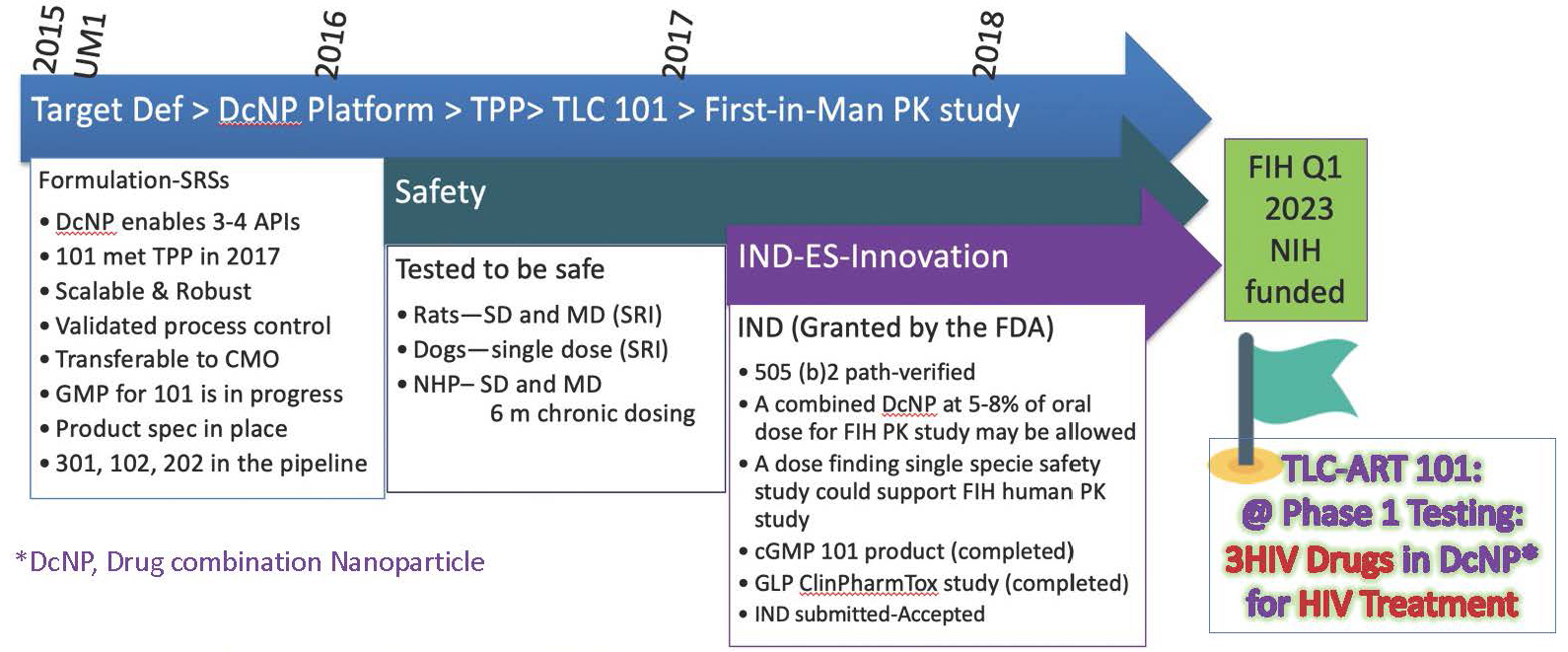
- Chose HIV treatment indication to advance U=U; uptake has also been considered; this work has been supported by public-private investment over past 7-8 years.
- Discovery of DcNP technology enabled the stable combination of 3 existing HIV drugs with disparate, incompatible properties.
- Determined how to assemble stable product, simplified the manufacture process, and transferred to CMO.
- IND enabling studies innovation – leveraged known efficacy and safety profile of existing HIV drugs.
- Submitted IND application for treatment indication to accelerate development.
- Shorter and smaller studies required than for prevention indication (24 vs 48 weeks) – once sustained viral suppression is proven, can move to prevention (i.e., 1.5 to 2-log reductions sustained over 24 weeks).
Investigational New Drug status of TLC-ART 101 – 3 HIV drugs (TFV/LPV/r) in one SC LA self-injectable product.
- Protease Inhibitor is useful for pediatrics and pregnant women, but HIV treatment has largely transitioned to integrase inhibitors.
- Leverage TLC-ART 101 product development and IND-enabling plans to inform TLC-ART 301 product development.
NextGen TLC-ART 301 combines TLD – the best global regimen for HIV treatment. A global funder has provided access to Dolutegravir, and we can begin to imagine “the moonshot.”
- Global Long-acting Development (GLAD) Project scope – transform short-acting TLD to LA TLD (supported by UNITAID and NIH).
- Use DcNP technology to combine TFV, 3TC, and DTG (incompatible properties) into stable DcNPs (TLC-ART 301) and synchronize fixed-dose combinations for collective exposures.
- Start with one-month exposures – one self-administered SC TLC-ART 301 injection to replace 30 TLD pills.
- Leverage DcNP technology used to form TLC-ART 101 to form a stable nanoparticulate TLD product at room temperature (TLC-ART 101 stable for at least 1.5 years).
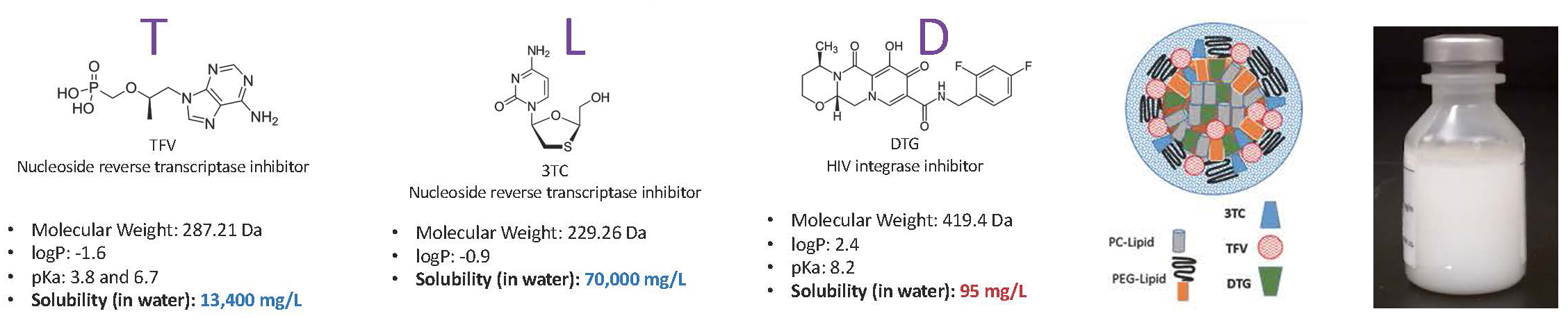
Lipid excipients used as the glue (not novel); Controlled solvent evaporation solubilization to form a stable DcNP TLD powder product (innovation); Re-suspension and size reduction for final product.
- Pre-clinical PK studies of single-dose SC TLC-ART 301 in NHPs show plasma exposures of TFV, 3TC, and DTG are sustained for 4 weeks (to be published).
- Need less drug in drug-combination and no delay to peak concentration.
- Leveraging PBPK modeling of TLC-ART 101 in NHPs (J of Pharm Sciences 2021), TLC-ART 301 targets integrase inhibitor to the lymphocytes and remains stable for sustained total exposures.
- Associated fraction of water-soluble and water-insoluble molecules is high throughout the body systems (TFV 99% and LPV/RPV 98%, respectively).
- 70% of dose is first loaded in and exposes the lymphatic system, then excess to the blood.
- Scaling to humans requires data from FIH TLC-ART 101 studies (NHPs have limited lymph nodes).
- Future – assess study outcomes and potential impact of TLC-ART 301 on sustained viral suppression for HIV treatment.
Summary
- We have achieved major milestones to reach Phase I treatment studies using TLC-ART 101, which has paved the way for development of TLC-ART 301 (TLD).
- TLD is in development – DcNP technology enables transformation of oral TLD to NextGen LA TLD – the first focus is HIV treatment, then prevention.
- We are looking for partners to develop NextGen LA TLD for global health benefit.
