Focus Group 1
Lessons learned from Islatravir toxicity
Polly Clayden: Rapporteur
Co-Chairs: Joseph Eron and Jeff Jacobson
Summary timeline.
- 2021 - Various ISL programs at different stages of development - different dosing intervals and routes of administration for HIV treatment and prevention
- Beginning of 2022 - Complete or partial hold across ISL programs due to lymphocyte toxicity observed in participants who received ISL
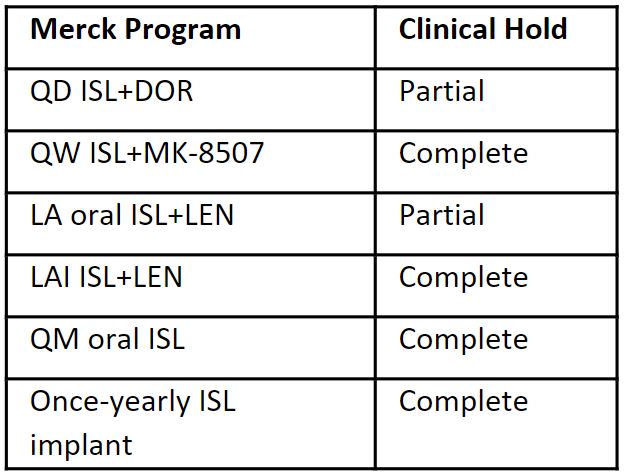
- 2022 - Merck comprehensive assessment to characterize the safety and mechanism of the lymphocyte effect.
- Not seen in animal, in-vitro, or Phase I studies.
- Isolated to lymphocytes and subsets (no effect on other hematologic cell lines).
- Exposure-dependent (mostly at 120mg QM).
- Likely related to high intracellular exposures, not mitochondrial toxicity.
- No change in infection risk compared to control arm.
- Phase 2 dose-ranging studies identified lower daily and weekly oral doses with efficacy and no lymphocyte toxicity – ISL (0.25mg) QD and ISL (2mg) QW.
- No dose identified for QM oral or injectable programs; implant on hold.
- New MK-8527 may have potential for prevention.
- Exposure-dependent (mostly at 120mg QM).
- Oral programs restarting in 1Q2023 – Merck QD oral program (ISL+DOR) and joint program with Gilead (ISl+LEN) using lower ISL doses (0.25mg and 2mg, respectively)
- Four studies of oral ISL+DOR QD (switch and treatment-naïve PLWH), including de-escalation for participants who received the ISL 0.75mg dose.
- Women who become pregnant during the study can remain on ISL after consent (as before); study population does not include highly-experienced PLWH.
- Phase 2 trial of oral ISL+LEN QW (PN045, 100 PLWH virologically suppressed on BIC/FTC/TAF).
- Four studies of oral ISL+DOR QD (switch and treatment-naïve PLWH), including de-escalation for participants who received the ISL 0.75mg dose.
Joseph Eron – “We’re all super-smart in retrospect.”
Lessons learned – considerations mostly focused on the ISL molecule vs broader applicable to development of LA products.
- Do we need a better animal model?
- Human cells are more effective than animal cells in phosphorylation of ISL.
- Accumulation of ISL-TP appears to be species specific.
- Do we need to bank cells? Would it have made a difference?
- Merck did not bank cells, as the lymphocyte effect was not expected.
- Could we develop another NRTTI that is not an adenosine analogue?
- Consensus that more attention should be paid to intracellular levels.
- Focus on classical pharmacodynamic principles.
- Took a long time to see toxicities – it is unrealistic to expect that we would know everything about a 6-month dosing interval from a 24-week study in people.
- Not concerned this will pause development of LA formulations.
- Not seen previously with CAB-LA or RPV-LA.
- Merck is committed to publishing all findings from the evaluations – lessons learned will be in the public domain.
What is the unmet need? What is the problem that needs solving?
- During last year’s LEAP, QW was considered less useful than QM dosing – once learned that QM is not possible, then QW becomes more attractive.
- Is the implant worth taking forward?
- Is post-natal prophylaxis a possibility?
- Pediatric indications.
- Merck is conducting extensive stakeholder consultations to identify unmet needs – a lot may be possible in terms of the science, but do people want and need it?
