Long-acting in-situ forming implants (ISFI) for TB and other infectious diseases.
Speaker: Martina Kovarova, Associate Professor, International Center for the Advancement of
Translational Science, Department of Medicine, Division of ID, UNC at Chapel Hill

Background
- LTBI (TB infection without symptoms) affects 25% of world population – approximately 2 billion.
- TB prevention therapy prevents LTBI from progressing to active TB and TBI in high-risk populations.
- Current therapies: 6- to 9-month INH QD; 4-month RIF QD; 1HP QD; and 3HP QW.
Development of LA formulations of anti-TB drugs.
- Focus on rifamycins (rifampin [RIF], rifapentine [RFP], and rifabutin [RFB]) due to potent bactericidal activity and ability to inhibit DNA-dependent RNA synthesis.
- Rifabutin selected as a model drug for development of an ISFI formulation.
- Reduced potential for DDIs – more suitable for TB-HIV coinfections.
- Higher tissue uptake due to high lipophilicity, larger volume of distribution, longer terminal half-life, lower minimum inhibitory concentration (MIC).
- Available as a low-cost generic medication.
- ISFI technology for a LA RFB formulation.
- Several FDA-approved products.
- A biodegradable implant is formed after direct injection – no need to remove but can be removed to stop drug delivery.
- Comprises three components that can be optimized – biodegradable polymer (PLGA), biocompatible solvent (DMSO/NMP), and the drug.
Analysis of ISFI formulation release properties in mice.
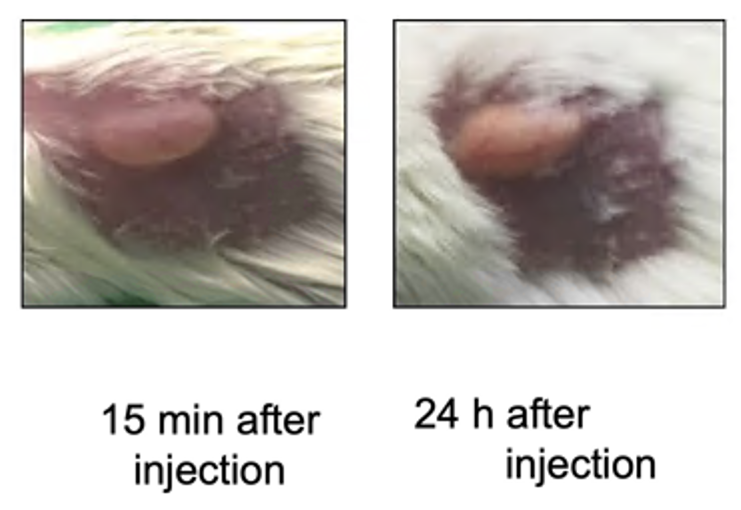
- After injection into the hydrophilic SC space, there is an exchange between solvent and body fluid, and the implant becomes solid.
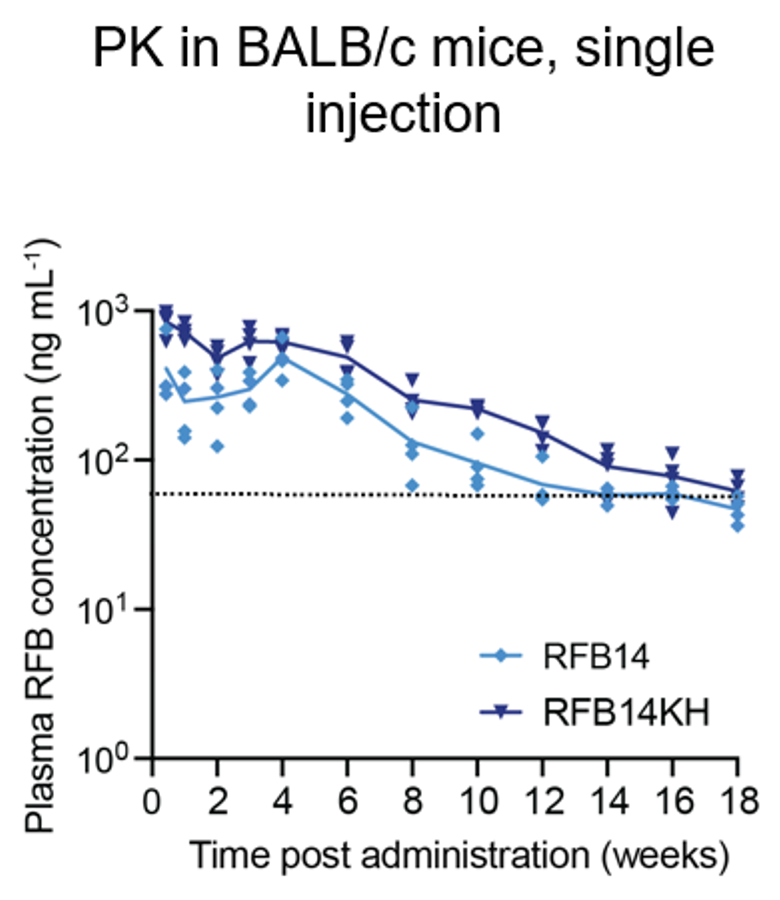
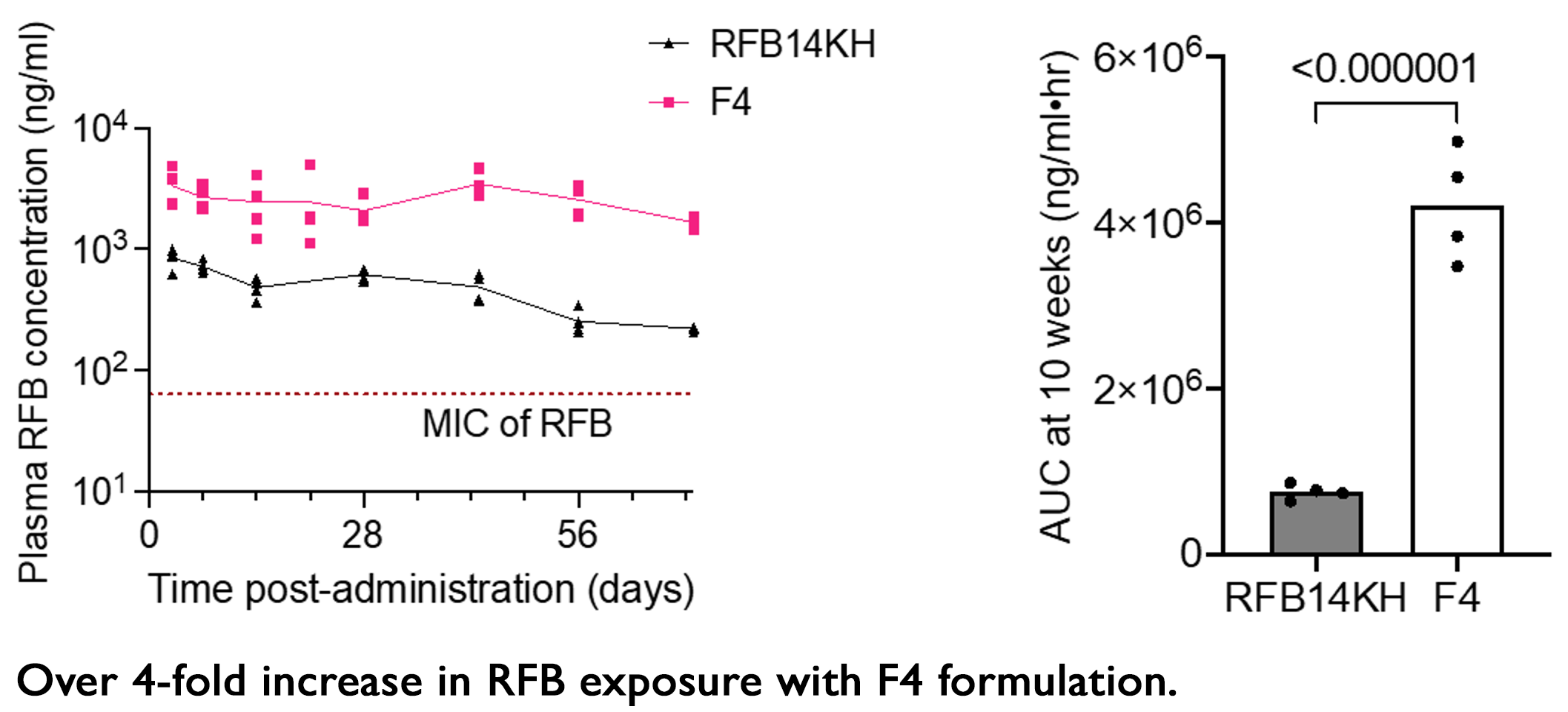
- A single-dose yields plasma RFB concentrations above MIC for 18 weeks.
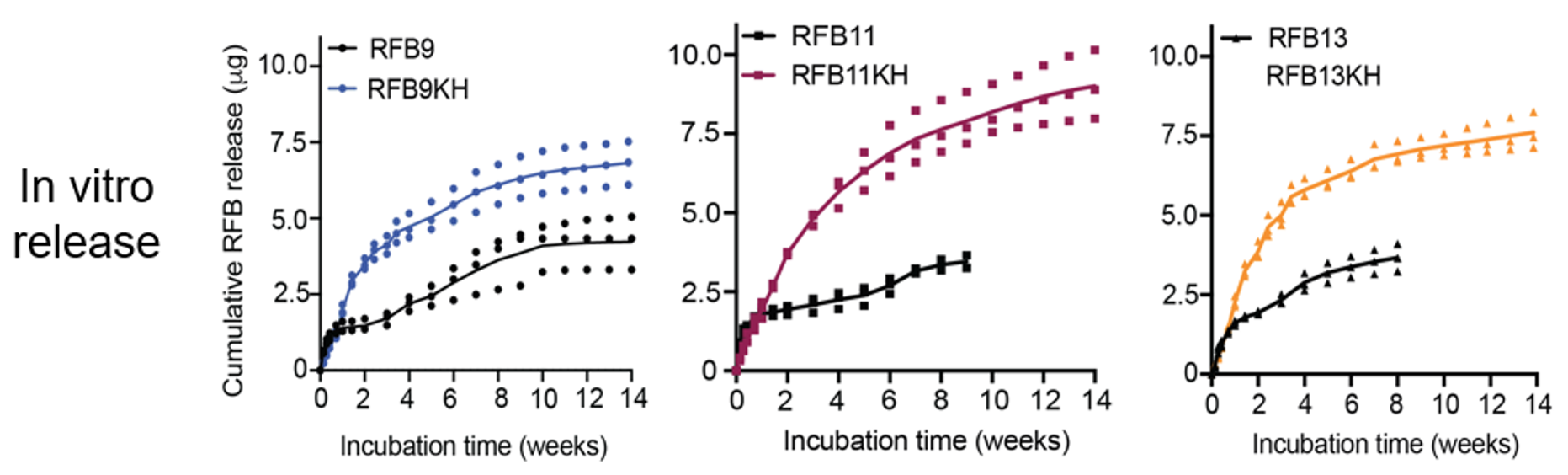
- Additives can increase RFB solubility and drug load (i.e., solubility in DMSO vs DMSO + additives) and improve release properties (in-vitro dissolution studies and in vivo studies).
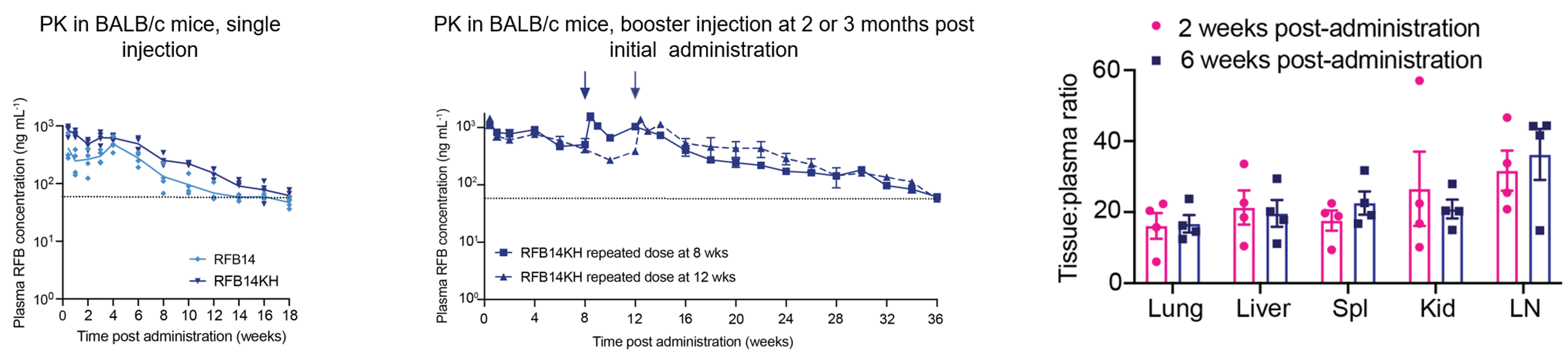
Evaluation of final optimized RFB-LA formulation (RFB14KH) in mice.
- PK and tissue penetration.
- SD + booster at month 2 or 3 – plasma RFB concentrations above MIC for 36 weeks (exceeds duration of all treatment options for LTBI).
- Better tissue penetration than previously published (nearly 20:1 lung to plasma ratio).
- In-vivo efficacy I (RFB14KH).
- pre-exposure treatment or placebo for 14 days, exposed to Mtb aerosol at day 0, and necropsy at day 1 through 28.
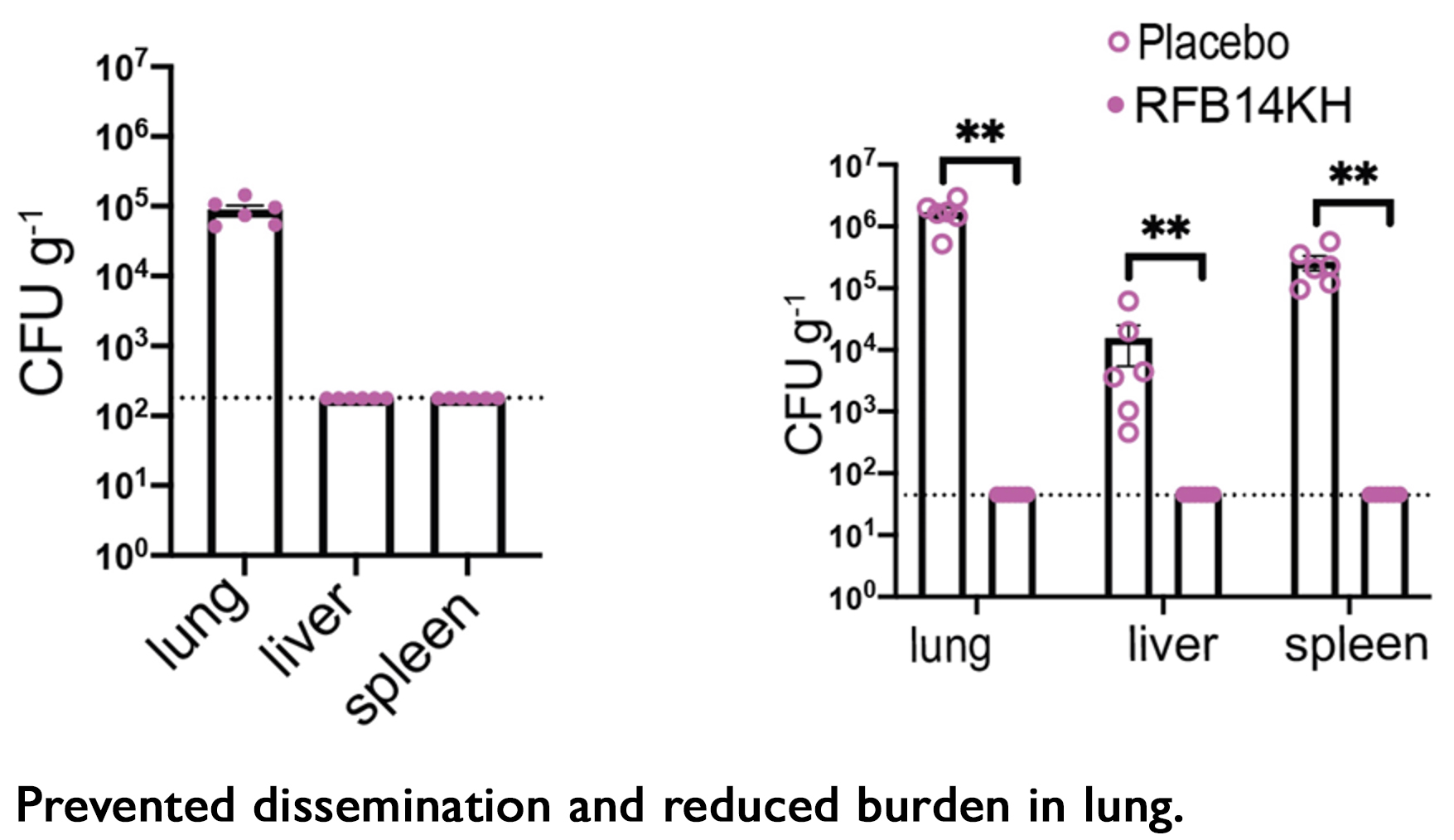
- All treated mice had bacterial load below level of detection (lung, liver, spleen).
- All placebo mice had high bacterial load (lung, liver, spleen).
- pre-exposure treatment or placebo for 14 days, exposed to Mtb aerosol at day 0, and necropsy at day 1 through 28.
- In vivo efficacy II (RFB14KH).
- Exposed to Mtb aerosol at day 0, post-exposure treatment or placebo at day 7, and necropsy at day 1, 7 and 28.
- Day 7– all mice have bacteria in the lung and no dissemination to liver or spleen.
- Day 28 – all treated mice had bacterial load below level of detection in lung, liver, and spleen; all placebo mice had high bacterial load in all three tissues.
Continued optimization efforts achieved increased drug load and plasma concentration after a single injection.
Summary
- We developed a LAI RFB formulation made of biodegradable polymer and biocompatible solvent that solidifies after SC injection.
- Addition of additives (amphiphilic compounds) increases drug solubility in organic solvent, allowing significantly increased formulation drug load.
- Translational relevance in mice.
- Delivered high plasma drug concentrations for 16 weeks without need for removal.
- Prevented acquisition of Mtb infection.
- Cleared acute Mtb infection from the lung and prevented dissemination to other tissues.
- A recently developed, optimized RFB-LA formulation (F4) delivers 4-fold more RFB.
