Islatravir update: next steps for the Islatravir (ISL, MK-8591) program
Speaker: Jay Grobler, Merck & Co.

Background on ISL.
- De-oxy adenosine analogue with a unique profile.
- Novel multiple mechanisms of action (NRTTI)
- High potency; strong resistance profile; generally well-tolerated in clinical studies; long half-life, and a favorable tissue distribution.
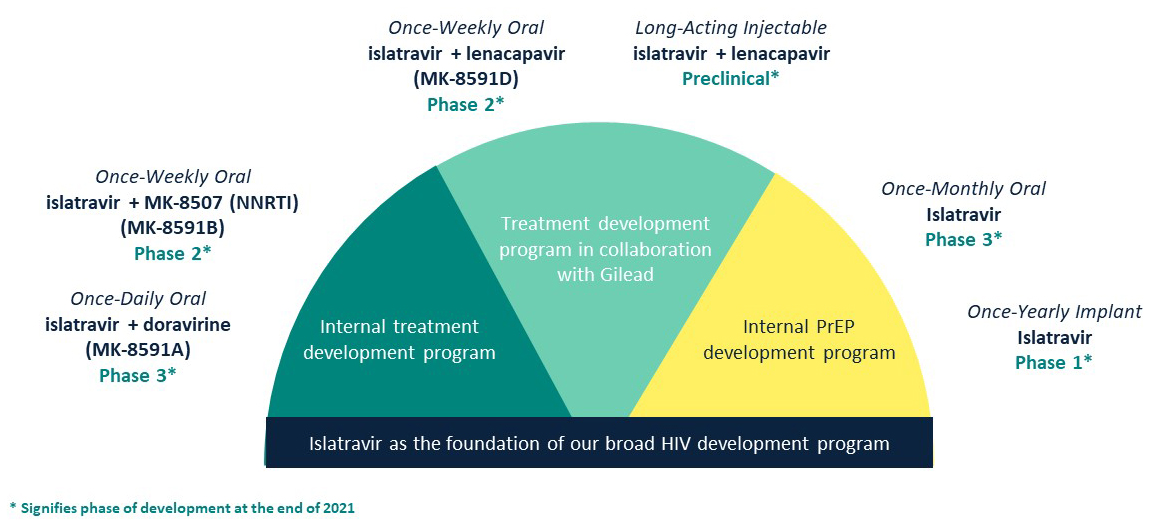
- Pursuing several ISL programs in 2021 for HIV treatment (daily and weekly oral and LAI 2-drug regimens) and prevention (monthly oral and yearly implant as monotherapy).
- Full (red) or partial (blue) FDA clinical holds were instituted across the ISL portfolio in Dec 2021 due to a lymphocyte effect (significant decreases in lymphocytes and CD4+ T-cells) observed among participants receiving ISL in late-stage clinical studies.
Comprehensive assessment of the lymphocyte effect completed in 2022 to characterize safety, understand the mechanism, and identify a pathway forward for ISL programs.
- Safety: 1) the magnitude of the lymphocyte effect is exposure-dependent (lower ISL doses are less likely to cause reductions); 2) other hematologic cell lines are not affected; 3) and there was no impact on incidence of infections.
- Mechanism: the lymphocyte effect is likely due to high intra-cellular ISL-TP exposures (or similarly high TP levels of other NRTIs), not mitochondrial toxicity.
- ISL doses were identified for daily (ISL/DOR) and weekly (ISL/LEN) oral HIV treatment.
- Exposure-response models for lymphocytes and CD4+ T-cells were used to find ISL doses expected to have low-to-no risk of lymphocyte effects and full efficacy.
- Unable to identify doses for monthly oral or injectable ISL formulations for HIV PrEP.
Phase 2 data and PK/PD modeling support evaluating a lower ISL dose in combination with DOR for daily oral HIV treatment (ISL 0.25mg/DOR 100mg).
- Phase 2b dose-ranging study of ISL (0.25, 0.75, and 2.25 mg) + DOR 100mg QD in treatment-naïve PLWH (PN011).
- ISL 0.25mg maintained high rates of virologic suppression through week 72, and efficacy was comparable to higher ISL dose groups.
- No ISL or DOR resistance variants were identified in any ISL dose group.
- ISL 0.25mg could mitigate the lymphocyte effect – Lymphocytes, CD4+ T-cells, CD8+ T-cells, B-cells, and NK cells were similar across ISL dose groups.
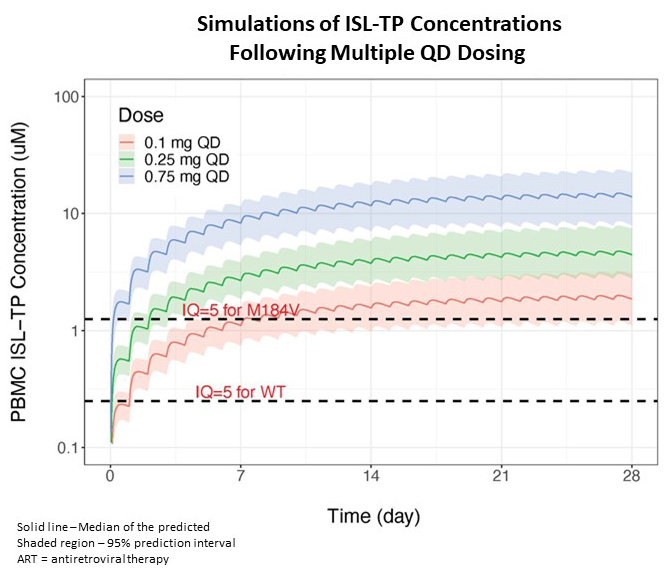
- Pooled PK/PD modeling of multiple daily dosing of ISL (0.1, 0.25, and 0.75 mg) predicts that 0.25mg will achieve therapeutic concentrations with increases in CD4+ T-cells that are similar to comparators at week 48.
Daily Phase 3 program for oral HIV treatment will restart in 2023 – four new trials evaluating a lower ISL dose (0.25 mg) + DOR 100mg in virologically suppressed (VS) “switch” and ARV-naïve populations.
- ISL/DOR vs baseline ART in VS PLWH – open-label, randomized switch (051).
- ISL/DOR vs BIC/FTC/TAF in VS PLWH – blinded, randomized switch (052).
- ISL/DOR vs BIC/FTC/TAF in treatment-naïve PLWH – blinded, randomized (053)
- ISL/DOR in VS participants enrolled in earlier clinical trials – open-label, single arm, dose de-escalation (054).
Weekly Phase 2 program was reinitiated in January 2023 with a trial evaluating a lower ISL dose (2mg) + LEN for oral HIV treatment.
- ISL/LEN Q1W vs BIC/FTC/TAF QD in virologically suppressed PLWH receiving BIC/FTC/TAF in the US (PN045); primary endpoint is virologic failure at week 24.
Summary
- Completed a comprehensive assessment of the lymphocyte findings, which has paved the way to restart daily and weekly ISL programs for oral HIV treatment.
- Daily Phase 3 program will restart in 1Q2023 with four new clinical studies using a lower ISL dose (ISL/DOR 0.25mg/100mg).
- Weekly Phase 2 program was reinitiated using a lower ISL dose (ISL 2mg + LEN), and the first participant was randomized in Feb 2023.
- Results from the Phase 2 daily program and modelling work will be presented at CROI 2023.
