Focus Group 3
Long-acting therapeutics for TB: planning and prospects for future clinical development
Ethel Weld: Rapporteur
Co-chairs: Kim Scarsi & Charles Peloquin
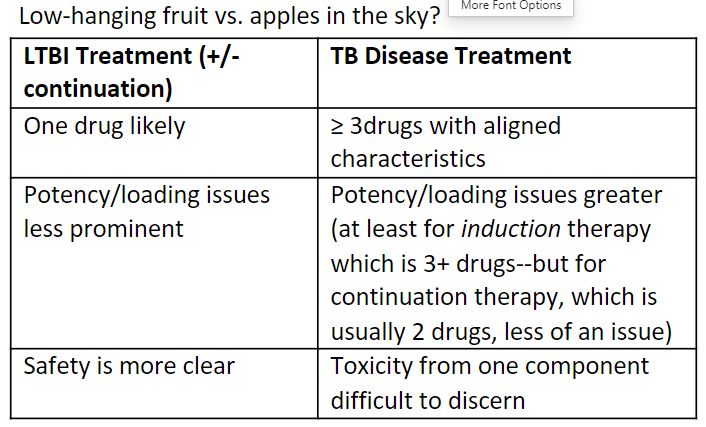
Considerations for LA Latent TB Infection (LTBI) treatment.
- RIF, INH, RPT have evidence as effective single agents, but especially when given for a longer course, may have adherence issues (e.g., <50% completion rates of 9H).
- 2-drug combinations are likely NOT needed to mitigate resistance risk – data shows rates of incident DR-TB disease after LTBI treatment are similar to background rates.
- Sterilizing activity may not be needed, although preferable (e.g., RIF, BDQ, Pa, DLM).
- INH took time, but worked – not a known sterilizer, but has good early bactericidal activity.
- Tissue levels are important.
- A plasma gradient will “drive the drug to the bug” (no active transporters, just active diffusion).
- Animal models are essential to understand where the organisms reside, and drug levels in that compartment (Cavity? Tissue?) and in proxy compartments (Plasma?) that are needed for efficacy.
Considerations for LA LTBI treatment in children.
- Youngest children (under 2 years) are highest priority – increased progression, higher risk of disseminated disease, and greater potential impact (in QALYs) of disease prevention.
- Are PK needs different in youngest children?
- TB lives in tissues (and plasma when disease is disseminated), and tissue is not serum
– are PKPD relationships the same?
- TB lives in tissues (and plasma when disease is disseminated), and tissue is not serum
- What we want to achieve may be different in children vs adults.
- Is there a specific drug that only works in young children? (i.e., one that did not work in adults), that is still worth investigating in kids?
- Most kids who develop TB did not receive LTBI treatment (lacked access). So if long-acting LTBI treatment expands their ease of access, then it is a worthy goal for this population, already neglected in terms of gross undertreatment for LTBI, globally.
- Is tolerability/acceptability of injections less of a concern in kids? (For the youngest kids, who commonly receive multiple vaccine injections at once, perhaps it is.)
- Do we need to evaluate acceptability in an age-de-escalation manner?
- PK should be evaluated in parallel to mitigate delays in treatment.
- Some technologies are easier in kids than adults (e.g., microarray patches and injection, as less mg amount means less area and less volume, respectively).
- Shorter duration makes rapid weight change less consequential for PK/clearance (Q1M or Q3M).
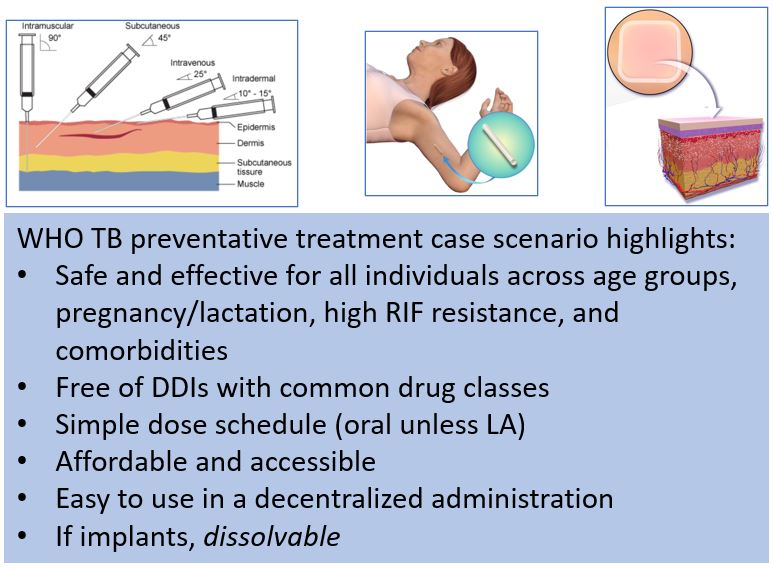
Desirable attributes of a LA TB medicine based on 2022 WHO TB preventive treatment case scenario.
What PK is needed?
- LTBI may require lower exposures than TB disease treatment because fewer bacilli in body – lower, more tolerable, more feasible volumes?
- Emulate the oral concentration? AUC:MIC?
- Stay above Ctrough of effective oral formulation?
- Could this be different for each drug? (e.g., in LTBI Balb-C 104 CFU mouse model: trough >3x MIC active for RBT, not for RPT)
- Stay in excess of MIC by 2- to 4-fold? (given known variability in determining MIC).
- Stay above Ctrough of effective oral formulation?
- Does concentration need to be > MIC for the entire dosing interval? Is high early concentration and lower later concentration ok? Is a long tail a concern? Less so for TB than HIV?
- Higher margin needed for possible resistant organisms?
- If bacilli are truly non-replicating, then resistance is not possible.
- Basic science info on Mtb under stress conditions would be useful.
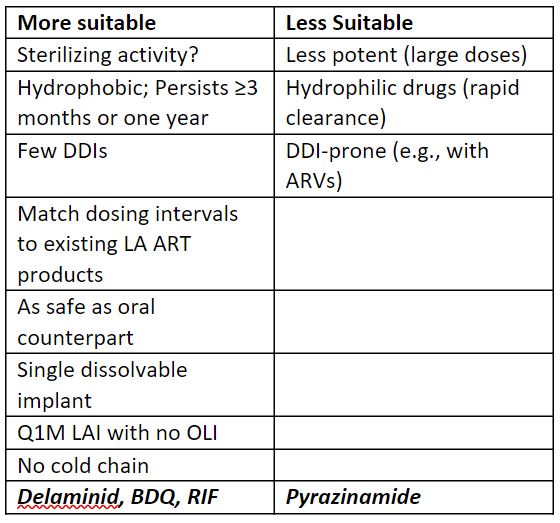
Critical pharmaceutical considerations for LA TB therapy.
potency, loading, physiochemistry, logP, rate of elimination, pro-drug approaches, volume, amphiphiles to alter solubility.
- Injectables – solid drug particle dispersions; microspheres; polymer approaches to controlled release of potent, water-soluble drugs.
- MAPs – minimally invasive, more acceptable, but how big must the patch be? Need push thru to POC.
- Implants – biodegradable (ISFI). Non-biodegradable, surgically implanted possibly more trouble than it’s worth for short-duration LTBI treatment? For TB treatment, don’t need all 3-4 drugs for the whole treatment duration, so tunability is needed.
- Hyaluronidase co-administration to allow larger injection volumes – impact on PK?
- Cold chain issue – easier to address with a solid product than liquid?
Is it worthwhile to look at new molecules with all the regulatory hurdles?
- Available therapeutics were chosen based on oral bioavailability, but lipophilic drugs with poor oral bioavailability are good for injectables.
- Target something FDA approved in another dosage form.
- Formulation fixes for stability issues; what are the goals? (e.g., RBT in solvent – stable for 3 months vs RBT formulated with polymer – no degradation at 2 years).
End-user preferences determine use.
- Need to assess preferences using interviews, focus groups, discrete choice experiments, and iterative processes to inform what products people will use.
- Who participates in the surveys of safety, efficacy, etc. matters.
- Target Regimen Profiles survey – one of 100 stakeholders with lived experience of TB.
- We need more rigorous patient preference work for TB drugs.
What are the regulatory challenges and solutions?
- Learn from LEAP Process, leverage modeling and simulation and approved LA formulations.
- Orphan drug designation for LTBI (based on low incidence of conversions to active TB in US)?
- Breakthrough therapy designation?
- RPV (11 years from P1 to clinical use) vs LEN (same journey was 4 years) because precedent was set.
- What level of known adverse drug reactions from an oral formulation is acceptable with a LA one?
- Is removability/reversibility mandated? Continual risk: benefit assessment.
- What level of data are needed to move from animals to humans?
Summary
- Goal is LA one-drug option for LTBI in all populations.
- Build expedited pathway for other indications.
- What does not work in adults may not work in children – need parallel development.
- Existing therapeutics preferred, but late pipeline should be considered.
- Continue to discuss appropriate targets – pre-clinical PKPD work and bridge to humans.
- Leverage learning from approved LA products in other disease states.
- Eligard, Sublocade, Perseris, Atridox, etc.
- Measure lived experience (stakeholders’ views).
- Balance regulatory issues.
- Public-Private partnerships essential.
“I will try to identify some of the questions, but I won’t promise the answer.”
