Long-Acting ART Program
Speaker: David Margolis, Head, Infectious Disease Therapy Area at Brii Biosciences

Brii Biosciences HIV program is focused on developing a weekly, oral single-tablet regimen (STR) for HIV treatment.
- Intended to support adherence and needs of PLWH who prefer an oral regimen over existing injectable LA ARV options.
- Modified-release (MR) LA RPV formulation (BRII-778) – potential for use as a partner molecule for a complete weekly oral regimen for HIV treatment.
- Leverages relatively low RPV dose and long intrinsic t1/2.
- Phase I complete.
- Proprietary pro-drug formulation of EFdA (BRII-732) – potential as a low-dose weekly oral tablet for HIV treatment and PrEP.
- Patent application published.
- Initial Phase I study complete; clinical hold lifted for conduct of Phase 1 dose-finding study.
Development of BRII-778 hinges on the ability to achieve and sustain therapeutic exposures within the safety window for the known QTc effect observed with RPV doses ≥75 mg QD.
- In silico human PK projections of potential BRII-778 dosing (200 to 300mg QW) and QD RPV.
- Need to delay and blunt the Cmax and delay the Ctrough to achieve therapeutic targets for QW RPV dosing while mitigating the QTc risk.
- QW formulations may have a differential QTc effect – relatively short time spent at Cmax compared to repeat QD dosing.
- Pre-clinical PK studies in dogs identified three MR formulations with distinct in vitro dissolution profiles.
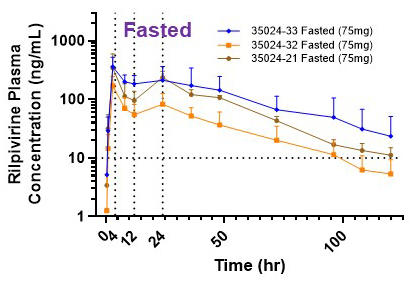
- A21 (slow), A32 (intermediate), and A33 (fast) vs immediate-release RPV.
- Phase 1 SAD studies across the selected formulations.
- High doses of A3 formulation achieve therapeutic targets for QW dosing, but the Cmax is above the QTc threshold – single 750 mg dose yielded Cmax> 700 ng/mL and maintained plasma RPV exposure above 60ng/mL at 1 week.
- Generally safe and well-tolerated – we observed a concentration-dependent QTc effect, but no individuals met QTc stopping criteria.
- Future directions.
- We achieved MR RPV formulations with differential absorption and dissolution rates (change in the Tmax from immediate-release RPV).
- Further optimization of the formulation is required.
The novel pro-drug, BRII-732 is efficiently converted to EFdA (ISL) in vivo with comparable exposures, efficient intracellular uptake and safety profile in preclinical studies.
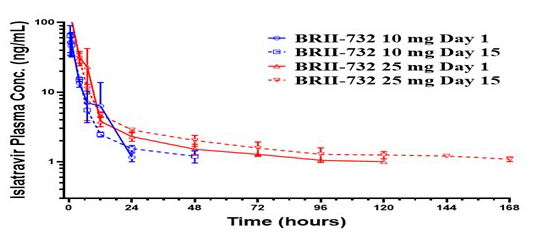
- Single and multiple oral administration of prodrug demonstrate linear PK and achieve therapeutic targets – Phase I randomized, double-blinded SAD (10mg to 200mg)/MAD (10mg and 25mg QW) of BRII-732 vs ISL in healthy volunteers. *designed prior to knowledge of ISL lymphocyte toxicity.
- Fast and efficient release of ISL from BRII-732 – no measurable systemic exposure to pro-drug following single or repeat dosing up to 200mg.
- Long half-life and dose-dependent PK profile with no meaningful accumulation in plasma after repeat dosing.
- Efficient intracellular ISL-TP formation in PBMCs in a dose-dependent manner – long cellular t1/2 and significant ISL-TP accumulation after 3 QW 25mg doses.
- Well-tolerated at single doses up to 200mg and repeat doses up to 25 mg.
- No AEs > Grade 1 and no lymphocyte effect at 10mg and 25 mg QW.
- Future directions
- Safety and PK data support further development of BRii-732 as part of oral weekly combination ART
- Ongoing development focused on selection of a low-dose QW tablet formulation
Conclusions.
- Weekly oral dosing is an important goal to provide options for PLWH.
- Current BRII-778 formulations are unable to achieve sufficient Ctrough exposures while remaining below the Cmax required to minimize the QTc risk.
- BRII-732 shows promise as a weekly oral agent.
- Phase I low-dose tablet study to begin 2Q2023 – dose to approximate daily ISL 0.25mg exposures (below the level where lymphocyte toxicity has been observed).
- A partner agent is required for a complete regimen for HIV-1 treatment – in discussion with multiple companies across classes of agents.
- Potential for use as monotherapy for HIV PrEP.
