Update from LONGEVITY
Speaker: Andrew Owen, Centre of Excellence in LA therapeutics (CELT), University of Liverpool

LONGEVITY aims to develop LA interventions for LMICs in malaria prevention (atovaquone alone or in combination), TB prevention (rifapentine in combination with a novel INH pro-drug) and HCV cure (glecaprevir/pibrentasvir combination).
- Drugs selected based on similarity to other successfully developed LAIs – focused on target plasma concentration, aqueous solubility, and plasma half-life of oral products in humans (pro-drug strategy needed for INH to reduce aqueous solubility).
- Have access to several particle processing technologies to develop LAI medicines (emulsion-templated freeze drying; emulsion templated spray drying; nanoprecipitation; high pressure homogenization).
Indication-specific challenges.
- Malaria – prevention of infection vs. disease.
- Difficult to assess active infection in persons living in endemic countries.
- Is a combination product needed to mitigate resistance risk?
- Conducted research on the transmissibility of atovaquone-resistant parasites.
- Potential for combination with a monoclonal Ab.
- Duration of the malaria season varies by region – need prevention for different periods of time (single vs multiple doses).
- Hepatitis C virus.
- Current oral combinations have high cure rates, but huge benefits of LA expected completion of therapy.
- Access to glecaprevir and pibrentasvir and intellectual property challenges.
- TB – prevention of active disease in people with LTBI.
- Is a combination product needed, particularly given the effectiveness of other preventive therapies?
- one-month rifapentine + INH is as effective as 9-month INH.
- Rifampicin already proven as a single agent for prevention
- Phase 3 Asteroid Trial is underway for rifapentine as a single agent
- Is a combination product needed, particularly given the effectiveness of other preventive therapies?
Structure of LONGEVITY activities and overview of progress with a focus on specific outputs.
- Research on the transmissibility of clinically relevant atovaquone-resistant parasites (Y268S) Preprint available online at https://www.biorxiv.org/content/10.1101/2023.02.07.527535v1.
- Fitness cost evident throughout lifecycle, and no detectable Y268S sporozoites in mosquito salivary glands.
- Multiple failed attempts at transmission to humanized mice using Y268S-fed mosquitoes.
- Provides evidence that resistance mutations cannot transmit with implications for a single-agent intervention.
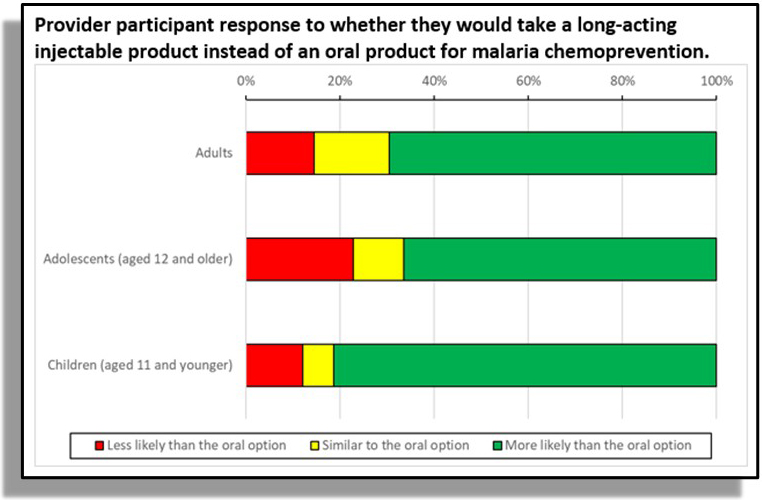
- Patient/provider attitudes survey for malaria.
- Most would “definitely try” injectable chemoprevention.
- Patients (80%) and parents/caregivers (84%).
- Providers: adult patients (70%) , adolescent patients (67%), and patients <12y (81%).
- No obvious preference regarding frequency of LAI.
- Patients: Q1M (52%), Q2M (41%), and Q3M (55%).
- Providers: Q3M (71%), then Q1M (63%).
- Very Beneficial” attributes of LA therapy.
- Patients: improved effectiveness (87%); easier to take (85%); fewer side effects (76%); and discretion (75%).
- Providers: all benefits rated high, particularly long-term malaria prevention (90%).
- Most would “definitely try” injectable chemoprevention.
- Preclinical PK studies of LAI glecaprevir/pibrentasvir in rats.
- Linear dose-dependent PK; achieved plasma exposures above the human oral Ctrough for 12 weeks (aiming for 8 weeks); similar hepatic-to-plasma concentration ratios between LAI formulations and oral delivery.
- Different release kinetics observed for glecaprevir (quick absorption) and pibrentasvir (slower release into systemic compartment)– likely due to differences in aqueous solubility.
“LONGEVITY is a huge team effort”
Unitaid
Univ of Liverpool
Johns Hopkins
University of Nebraska
CHAI
Tandem Nano Ltd
TAG
MPP
Queen’s University Belfast
LEAP
