Targeted Long-acting Combinational Anti-Retroviral
Therapeutic (TLC-ART) Program – Update
Speaker: Rodney Ho, Professor of Pharmaceutics and adjunct Professor of Bioengineering at University of Washington

Reviewed the Global Long-Acting Drugs (GLAD) project and shared progress on study outcomes and global implications.
TLC-ART GLAD Project develops LA drug combinations for global health impact.
- Aim – transform short-acting once-daily oral TLD tablets into a long-acting once-monthly SC TLD injection (LAI-TLD) to achieve viral suppression for HIV treatment (all-in-one cART administered via a single once-monthly SC injection replaces 30-90 oral tablets).
- Approach leverages innovations to accelerate R&D timeline to global launch.

- TLC-ART’s enabling technology – DcNP can combine up to 4 existing drugs with disparate physical properties into a single injectable suspension (simple, scalable, transferable technology).
- New private-public funding paradigm – Unitaid plus NIH funding is intended to accelerate pre-clinical development of LAI-TLD to first-in-human studies.
Why develop DcNP formulations of existing HIV drugs?
- Enables “all-in-one cART” via a single SC injection (e.g. LAI-TLD) vs combining single-agent dosing forms (e.g. LAI CAB + LAI RPV).
- Adjustment of drug ratio is not as flexible, but DcNP could enhance patient acceptability (one injection vs separate single-agent injections) and achieve higher HIV clearance in cells.
- Extends the half-life of short-acting oral drugs.
- Single-drug DcNP formulations of TFV, DTG, 3TC, RTV and LPV have a longer apparent plasma half-life (hundreds of hours in NHPs) than oral dosage forms (hours to days in humans).
- Targets all drugs in the formulation to HIV host cells and tissues (data from LPV/RTV/TFV DcNP).
- Enables LA and higher multi-drug levels in lymph nodes and PBMCs compared to plasma (lymph nodes > PBMCs > plasma).
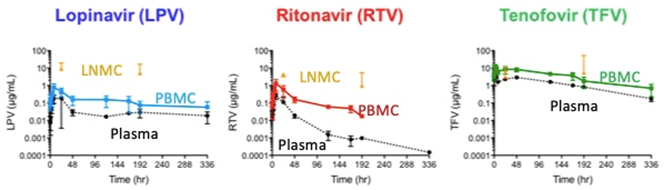
- A majority of DcNP dose (~70%) is quickly localized from the injection site to lymph nodes and retained for enhanced exposure (first pass, slow clearance); excess DcNP (~30% of dose) enters the blood (PBMC > plasma, intermediate clearance).
- Enables higher PBMC:plasma ratio than is possible with oral cART – DcNP injection in NHPs vs oral tablet in humans: LPV (4.02 vs 0.27); RTV (7.40 vs 0.52); and TFV (3.01 vs 0.65).
- Enables LA and higher multi-drug levels in lymph nodes and PBMCs compared to plasma (lymph nodes > PBMCs > plasma).
Progress in DcNP cART platform.
- Five LA cART candidates validated in NHPs (including TLD) – LA PK; cell:plasma ratio >1 for all drugs in combination; and basic safety; LPV/RTV/TFV DcNP is entering first-in-human studies.
- DcNP technology has been licensed for global use through Medicines Patent Pool (MPP) – MPP-UW-Unitaid partnership announced Dec, 2021.
- TLD DcNP formulation is stable and scalable – single- and 2-drug (TD, TL) combinations may be feasible; several triple-drug (TLD) formulations are being evaluated for LA PK profile in NHPs.
- Manufacturing process has been simplified to scale – eliminating the need to remove unbound drug reduces cost (based on MBPK and PBPK study data).
- Conducted modeling simulations of LPV/RTV/TFV DcNP formulation.
- MBPK modeling – water-insoluble (LPV and RTV) and water-soluble drugs (TFV) remain associated with DcNP in vivo.
- PBPK modeling (free-drug mixture vs DcNP formulation) – DcNP-bound drugs are retained in cells in lymph nodes (leading to the targeting and LA PK outcomes); the model can validate and project PK time-course for tissues and nodes of interest.
Estimated potential global impact of TLD as a LA injectable – CEPA outcome projections.
- Transition from oral TLD to LAI-TLD could gain 2.3% in viral suppression among PLHIV on ART by 2030 (assuming 100% oral-to-LAI TLD transition starting in 2025).
- LA reduces non-suppression due to treatment disruption by 75%.
- Potential for LA to gain fast-track targets with: improved clinical outcomes; well-tolerated by PLHIV; and costs comparable to oral formulation.
Summary and Next Steps.
- Significant progress in the transformation of short-acting oral TLD to LAI-TLD – at the proof-of-product concept stage and moving towards market (preparing to improve patient acceptance and adherence and implementation science).
- DcNP platform has the flexibility to adapt if the field moves to more potent product compositions.
- We continue to seek supporting partners to improve outcomes and impact of the project.
