Lenacapavir (GS-6207): A First-in-Class Long Acting HIV Capsid Inhibitor
Speaker: Martin Rhee, Director of Clinical Research at Gilead Sciences

Review of Lenacapavir (LEN) and ongoing clinical studies for HIV treatment
- LEN is a potent ARV (EC50 50-100pM) due to multiple HIV replication targets (inhibits nuclear transport, capsid assembly and virus assembly and release)
- Capella – Phase 2/3 study among 72 heavily treatment-experienced PWH with multi-drug resistance (resistance to ≥2 agents from 3 of 4 main ARV classes, and ≤2 fully active agents from 4 main ARV classes) or failing current regimen (HIV-1 RNA ≥400 copies/mL)
- Dosing periods – Functional monotherapy period (initial 14 days) and Maintenance period (26 weeks).
- Study cohorts: Randomized cohort (LEN oral [n=24] or placebo [n=12] + failing ARV regimen x 14 days, then LEN SC q6mo + OBR x 26 weeks) and Non-randomized cohort (repeat testing confirmed HIV-1 RNA <400 copies/mL or decline ≥0.5 log10 copies/mL) LEN oral + OBR x 14 days, then LEN SC q6mo + OBR x 26 weeks.
- Calibrate – Phase 2 open-label, randomized study among 182 treatment-naïve PWH (HIV-1 RNA ≥200 copies/mL and CD4 ≥200 cells/µL).
- Dosing periods: Induction period (26 weeks) and Maintenance period (26 weeks).
- Treatment groups: Groups 1 and 2 (LEN SC q6mo + F/TAF oral x 26 weeks, then LEN SC q6mo + TAF or BIC x 26 weeks); Group 3 (LEN oral + F/TAF x 52 weeks); Group 4 (B/F/TAF x 52 weeks).
Capella – week 26 data suggest that combination LEN achieves virologic suppression, robust CD4 recovery and is well-tolerated in our treatment-experienced population with drug-resistant (NRTI 99%; NNRTI 97%; PI 81%; INSTI 69%), advanced HIV disease (64% with CD4 ≤200 cells/µL).
- Viral load declined by nearly 2-fold during functional LEN monotherapy (LEN -1.93 vs Placebo -0.29, p<0.0001) (results from randomized cohort, n=36).
- Over 80% had HIV-1 RNA <50 copies/mL, and mean CD4 change was +81 cells/µL over 26-week maintenance period (results from randomized cohort, n=36)
- 11% of randomized cohort had emergent LEN resistance at 26 weeks – all 4 cases were at high risk (2 had no other fully active agent and 2 had poor adherence to OBR)
- Safety (overall population, n=72): No SAEs related to LEN; <10% reported AEs related to LEN (N and D were most common); 13-25% had ISR related to LEN (swelling, erythema, pain, nodule and induration were most common – most grade 1 and lasted days/nodules lasted weeks to months).
Calibrate – data from the 6-month Induction Period suggest that combination LEN may achieve rapid virological suppression with low emergence of LEN resistance in treatment-naïve PWH.
- > 90% of LEN treatment groups had HIV-1 RNA <50 copies/µL at week 28 (Group 1 [n=52] 94%, Group 2 [n=53] 92% and Group 3 [n=52] 94% vs B/F/TAF [n=25] 100%).
- LEN performed as well as B/F/TAF over the first few weeks (B/F/TAF is known to achieve fast virologic suppression).
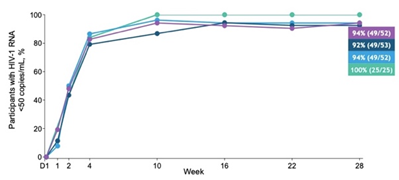
- One participant had emergent LEN resistance at week 10 (in Group 2) – resistance testing performed if HIV-1 RNA ≥50 copies/mL and <1log10 reduction from Day1 to week 10
- Plasma LEN concentrations were consistently within target range, and mutations in CA (HIV capsid protein, Q67H+K70R) were preceded by RT (reverse transcriptase, M184M/I), suggesting poor adherence to F/TAF likely led to LEN resistance.
Summary and Next Steps.
- LEN as part of a combination regimen for HIV treatment (Capella and Calibrate) –
- LEN led to high rates of virologic suppression in heavily treatment-experienced PWH with multi-drug resistance (LEN + OBR) and treatment naïve PWH (LEN + F/TAF) and was well-tolerated
- Both studies are ongoing – one-year data will be presented at CROI 2022 (Ogbuagu O et al one-year Capella data; Gupta S et al one-year Calibrate resistance data).
- LEN for HIV prevention –
- Two Phase 3 PrEP studies are ongoing (Purpose-1 and Purpose-2)
“we are very excited about the potential of LEN for treatment and prevention of HIV to address the diverse needs of people with HIV or people at risk”
