Marc Baum, Senior Faculty of Organometallic & Environmental
Chemistry at Oak Crest Institute of Science
Speaker: Tenofovir implants and local toxicity: what have we learned to date?

Shared findings from preclinical studies and perspectives on this debated topic.
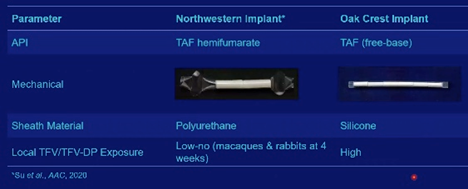
Oak Crest and Northwestern University (NWU) have each developed a tenofovir alafenamide (TAF) implant – both are reservoir devices filled with tablets containing the active pharmaceutical ingredient (API), but have different designs, are made of different materials and have conflicting local tissue safety.
- TAF is a highly potent pro-drug of tenofovir (TFV) and one of few candidates with enough potency to be formulated and delivered via subdermal (SD) implant.
- Oak Crest vs NWU TAF implant.
Preclinical Studies of the Oak Crest implant in dogs, mice and sheep suggest that the target human dose (TAF release rate of 0.25mg/day per implant) should only lead to a minimal foreign body response (mild inflammation and capsule formation).
- TAF release rate < 1mg/day led to very mild inflammation associated with a foreign body response in dogs (TAF release rates ranged from <1mg/day to >7mg/day over 14 days or 30 days); significant inflammation was observed at release rates above 1mg/day and worsened at release rates above 1.5mg/day.
- Only observed the expected foreign body response across a range of release rates over relatively short durations in mice and sheep (up to 0.6mg/day for 28 days in mice; up to 0.3mg/day for 14 days in sheep).
Placebo-controlled studies of the NWU implant in macaques suggest concerning local toxicity, possibly due to a drug effect.
- Each animal served as its own control (active and placebo implants were placed contralaterally).
- Active implant with TAF release rate of 0.13mg/day led to unacceptable inflammation and severe necrosis in some cases (local effect assessed at 30 days [n=2] or 90 days [n=4]).
- No local toxicity observed around placebo implants.
Differing TFV and TFV-DP exposure in tissue surrounding the Oak Crest and active NWU implants suggests that contrasting local toxicity may involve more than a simple drug effect.
- Compared local tissue concentrations of TFV and TFV-DP from existing pre-clinical datasets in mice and sheep (Oak Crest implant) and rabbits and macaques (active NWU implant).
- Oak Crest implant led to high TFV and TFV-DP exposure at the implant site (TFV-DP exposure was ~100-fold lower than TFV in both animal models).
- Active NWU implant led to: 1) low to no TFV and TFV-DP exposure at the implant site in rabbits at 4 weeks and macaques, and 2) a wide distribution of TFV exposure that spanned Oak Crest values with no TFV-DP exposure in rabbits at 12 weeks.
- What else could be at the implant site and cause a local reaction in the case of the active NWU implant?
- Different API formulation, different device shape, different device material.
- Oak Crest MALDI mass spectrometry studies show that all intermediate compounds produced during TAF metabolism (metabolite Y, metabolite X, TFV, TFV-MP and TFV-DP) are seen in tissue sections collected at the implant site and their distribution around the implant varies.
Next Steps
- Continue to investigate why some research groups are seeing TAF implant toxicity and others are seeing much less.
- Proceed with Phase 1/2 clinical trials to assess the safety, acceptability and PK of the Oak Crest sustained-release TAF SD implant for HIV prevention in women (CAPRISA 018)
- Phase 1 component ongoing (dose and duration escalation among low-risk women in South Africa) – most women have had implant for 6 months; 3 safety reviews completed; DSMB recommends continuation of trial.
- Phase 2 component planned to assess extended safety, tolerability and acceptability (randomized 1:1 TAF SD implant + oral placebo vs placebo implant + oral TDF-FTC)
“TAF still has a lot of potential, especially as the list of potent ARV drugs that are useful for implants is dwindling – we really do need to give TAF the full benefit of scientific investigation”
