DAIDS Preclinical Services Program to Accelerate Drug Development
Speaker: Keith W Crawford, Health Scientist Administrator at Division of AIDS,
National Institute of Allergy and Infectious Diseases, NIH

Provided a program overview with a focus on available services under Preclinical Pharmacology and Toxicology and Formulation Development and Manufacture of Clinical Dosage Forms.
The DAIDS Preclinical Services Program is a valuable resource for investigators developing next generation therapeutics for HIV and related co-infections – high priorities include longer acting ARVs, novel targets and inhibitors and novel immune-based therapies.
- Provides preclinical support to advance promising therapeutic candidates towards clinical trials (fills critical product development and resource gaps in investigator programs).
- Investigators receive products, data and specialized expertise from NIAID contractors at no cost to the investigator (investigators do not receive NIAID funding).
- Available services fall under 6 technical task areas spanning product development from initial drug discovery and lead optimization to preclinical development, including IND-directed studies and activities required for regulatory submissions and clinical trials.
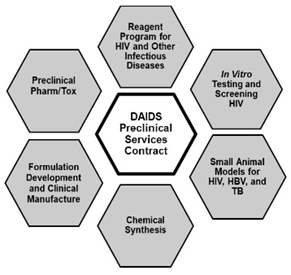
Example task area 1: Formulation Development and Clinical Manufacture – available services (all studies conducted in compliance with cGMP).
- Develop new formulations to enhance product solubility or bioavailability
- Develop alternative dosage forms (different strength or route of administration).
- Develop and validate analytical assays to determine identity, strength, quality, purity, stability, and drug release methods.
- Develop manufacturing processes and procedures.
- Prepare reports to be included in the Chemistry, Manufacturing and Controls (CMC) section of regulatory submissions.
Example task area 2: Preclinical Pharmacology and toxicology – available services are directed towards meeting requirements to be included in the IND submission (all studies conducted in compliance with cGLP).
- IND-enabling studies – characterize in-vitro properties (ADMET, protein binding, bioavailability and bioequivalence, potential drug interactions); pharmacology in animals, toxicology (acute, repeated dose and chronic toxicity), safety analyses in different organ systems.
- Develop bioanalytical methods and perform bioanalytical studies.
- Prepare all required study reports for the IND package.
How to access services (www.niaid.nih.gov/research/daids-services-program-accelerate-drug-develo...).
- Submit a written request for services (specific needs, data package to support the request and overall product development plan)
- Requests evaluated internally with a team of expert scientists according to the following criteria: 1) matches NIAID priorities; 2) soundness of development plan; 3) investigator commitment (preliminary data, concurrent studies, communications with FDA); 4) ability of NIAID contract resources to fulfill requested services; and 5) availability of funds.
- If approved, NIAID point of contact issues a Material Evaluation Agreement (MEA) and coordinates transfer of products and data between investigator and NIAID contractor.
- Resources and services listed on NIAID website with contacts.
Past projects by task area.
- Clinical Dosage Forms Manufacture – novel formulation, delivery system or route of administration of an approved product that alters the PK.
- Repackaged nevirapine tablets into blister packs with a 48-month stability study.
- Manufactured methotrexate capsules and placebo with a 60-month stability study.
- Process development and GMP manufacture of a proprietary lipid-based product (manufactured a lipid nanoformulation).
- Preclinical Pharmacology and Toxicology.
- Pharm/tox studies of proprietary ARV nanoformulation for IND filing.
- Safety and pharmacology studies (GLP and non-GLP) of novel formulations of existing drugs (injection, oral, and inhaled delivery in rats and dogs).
- 6-month tox/TK study of sutezolid for mycobacterial infection.
- Repeat toxicity and TK studies of a novel ARV formulation in mice.
- Reproductive tox studies of a clinical stage ARV in rats (GLP, segment I/II).
- In-vitro mitochondrial tox studies.
