Update on ISL Development
Speaker: Jay Grobler, Associate Vice President of Infectious Diseases and Vaccines at Merck
Focused on the safety information emerging from the clinical trials of Islatravir (ISL).
Overview of ISL and studies.
- ISL is a nucleoside reverse transcriptase translocation inhibitor (NRTTI) with potent antiviral activity, a differentiated resistance profile compared to nucleosides currently approved for HIV treatment or prevention, and PK properties that support the potential for extended-duration dosing.
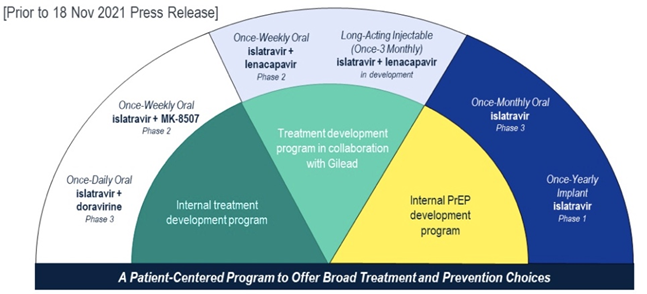
- ISL development program for HIV treatment (Internal and Merck-Gilead Collaboration) and PrEP.
Emerging safety information for ISL and MK-8507 during a Phase 2b stable switch trial evaluating oral ISL+MK-8507 for HIV treatment among adults virologically suppressed on BIC/FTC/TAF.
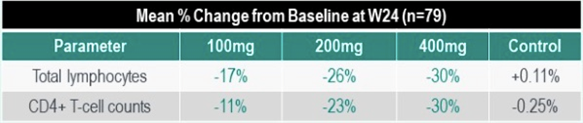
- Routine, blinded medical monitoring observed downward trends in total lymphocytes and CD4+ T-cell counts in a majority of participants at week 12 and 24.
- Mean % change at 24 weeks appears to be proportional to the MK-8507 dose (100mg, 200mg, 400mg), but still observed in the lowest dose administered.
- External data management committee (eDMC) recommended that Merck stop dosing and monitor participants – Dear Investigator Letter (DIL) sent to trial sites on 18 November 2021.
Merck Internal review of all ISL or MK-8507 trials (as of 18 November 2021) – hematological parameters reviewed to evaluate for similar trends in lymphocytes and CD4+ T-cells across all indications and dosing regimens.
- HIV treatment – stable switch Phase 3 trials of daily oral ISL+DOR in virologically suppressed participants showed <1% mean decreases in CD4+ T-cell counts at week 48 with no clinical adverse events (AEs) related to infection. Treatment eDMC recommended that Merck continue trials as currently designed.

- PrEP – Phase 2 study of ISL 60mg vs ISL 120mg vs placebo in HIV-1 uninfected participants at low risk of HIV-1 infection showed dose-dependent mean decreases in lymphocyte counts at week 24 that were in the normal range with no increase in clinical AEs related to infection. Prevention eDMC recommended that Merck continue ISL PrEP trials as currently designed (based on above Phase 2 trial data and Phase 3 trial data reviewed on 07 October 2021).

US FDA places clinical holds on clinical investigations under the following new investigational drug applications based on the aforementioned data (13 November 2021).
- Full clinical hold (stop dosing, increase monitoring and no further enrollment).
- o ISL oral and implant formulations (PrEP).
- o ISL injectable formulation (HIV-1 treatment and PrEP).
- Partial clinical hold (continue dosing those on study, but stop screening and enrollment).
- o ISL+DOR oral daily formulation (HIV-1 Treatment).
Summary and Next Steps.
- Status of Merck ISL Development Program – most programs in our portfolio have been impacted to varying degrees as of 27 December 2021 per FDA.
- Continue monitoring participants still receiving ISL.
- Investigate the underlying mechanism that lead to the decreases in lymphocytes.
- Evaluate the PK, safety and hematology from our clinical studies to understand the PK/PD relationship for this effect.
“We remain committed to the development of LA HIV Treatment and Prevention regimens AND we remain committed to fully understanding the potential paths forward for ISL.”
