Focus Group 1 - Developing LA formulations for treatment and prevention of HBV an HDV: Where are we now and where do we need to go?
Clinical and Public Health Needs
LA therapy is convenient and could prevent HBV reactivation stemming from poor adherence.
- Current HBV therapies are effective, but adherence drops off with daily long-term therapies. Clinical scenarios for LA HBV treatment.
Clinical
Jordan Feld (University Health Network) reviewed clinical scenarios for LA HBV treatment.
- During pregnancy for PMTCT.
- Oral medications can be challenging during pregnancy; a one-time LA dose would be beneficial.
- In the setting of immunosuppression.
- HBV reactivation is a life-threatening complication of immunomodulatory therapies (i.e., cancer chemotherapy and biologics).
- Avoiding daily HBV therapy where missed doses could have severe consequences would be helpful.
- Rural/remote areas.
- LAIs are particularly suited to high burden areas where care is intermittent or unavailable (i.e., SSA and many parts of Asia).
- LAIs, even with current therapies, would be a significant benefit.
- HBV cure.
- Delivering a stable backbone without interruption is important to achieve cure with a purely antiviral approach (backbone of current NRTIs is likely).
- If immunomodulatory therapies are added, need to ensure the safety of no interruptions.
- Pediatrics.
- Children are not always willing or able to take pills.
- HIV-HBV co-infection.
- Including HBV-active drugs in a LA HIV approach could control both conditions at the same time.
- Other potential areas exist.
Public health considerations
- An estimated 257 million people are living with chronic HBV worldwide – large market from a pharmaceutical perspective.
- Most new cases occur in infants at birth (MTCT).
- Birth dose HBV vaccination is a practical and effective PMTCT approach, but only 39% of infants born to HBV+ mothers received a birth dose vaccine in 2015.
- This number may be hard to raise due to the number of children born in non-traditional settings.
- Giving a LA agent during pregnancy could prevent some of these MTCT events – global health level.
Discussion Highlights
Chari Cohen from Hep B Foundation (represents patient/community voice).
- Daily pills can be stigmatizing or empowering - depends on the individual.
- Systemic access and discrimination are a concern for any non-curative treatment in RLS.Any treatment needs to lead to surface antigen negative status to be widely accepted – cannot get employment without seronegativity.
- It will be a challenge to deliver LA medications during pregnancy (due to access to care)
U=U campaign for HBV could be powerful in addressing discrimination/stigma.
LA HBV in pregnancy: in RLS, home deliveries are really the issue limiting the current PMTCT strategy.
- Many pregnant women attend at least one ANC visit (even if they deliver at home).
- Could implement a system – confirm HBV viremia via POC test and administer a LAI in the same ANC visit (would need to establish safety and same benefit as birth dose vaccination).
- As an add on, there is a need to train and license midwives to administer HBV birth dose vaccination at home deliveries.
Review of Existing LA HBV Efforts
TAF implants Marc Baum (Oak Crest Institute)
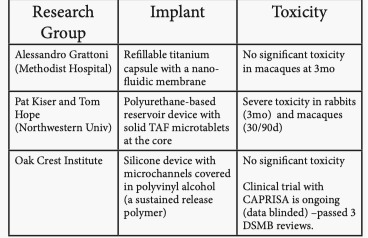
- TAF is one of few drugs potent enough to theoretically enable drug delivery up to 6mo from an implant.
- Overview of technologies being studied.
- Toxicity of Northwestern implant is possibly due to a combination of factors – mechanical, polyurethane material, fumaric acid plus TAF and wound healing.
TFV ProTides – Benson Edagwa (UNMC)
- Single IM injection of a novel TFV prodrug formulation (NM1TFV) suppresses HBV replication for up to 4mo in HBV-infected, humanized mice.
- NM1TFV gets distribution in lymph nodes and hepatocytes without specific organ targeting.
- Prodrug formulations are well-tolerated (preliminary toxicology and injection site examination).
- Nanoformulated TAF (control) administered at the equivalent IM dose had a minimal effect on HBV replication.
- Changing from oral to parenteral route of administration affects TAF biodistribution and delivery into the liver.
TFV prodrug bolus approach – Arnab Chatterjee (Calibr)
- Finding the right form of TAF and the right way to deliver it is key.
- Observed different release rates and differences in conversion to TFV-DP among oil-based vs aqueous suspensions of TAF – better results seen with free-base form compared to heavy fumarate.
- IM bolus of TAF (aqueous suspension) in dogs sustained good drug levels in PBMCs (TFV and metabolites) over 80 days.
- Good shelf stability for LMICs (potentially up to 6 months).
- Bolus approach allows ISR to resolve vs continual release strategies – still have work to do with histopathology.
Discussion Highlights
Are treatment and prevention targets for intracellular TFV-DP the same for HIV and HBV?
- PK targets for HBV treatment may be higher than HIV.
- Small study of patients with HIV-HBV coinfection: TFV levels were consistent with four doses/week and suppressed HIV, but not HBV.
- HBV is replicated exclusively in the liver. Without oral delivery, the advantage of first pass metabolism is eliminated –need to target the tissue.
- There are no strategies for targeting the liver with parenteral therapy.
- TFV ProTides have high drug levels in lymphocytes and liver tissue – enough to suppress HBV in mice, but do not specifically target the liver. Therapeutic concentration may have to do with the lipophilicity of the formulation.
- Rodney Ho also has evidence that TFV is taken up in lymphocytes and liver.
- Combining HIV prevention with a TFV product and HBV prevention in pregnancy would be useful.
- Meg Doherty (WHO): There is great public use for this work. There are many places where birth dosing is not happening – LA TFV for HBV and LA PrEP would be a nice correlate. How long from this stage to human to reality?
Need to engage industry to accelerate development.
- 257 million people represents a huge potential market for a LA formulation – large enough to offset small profits on an individual basis.
- This group can engage in consciousness raising with the pharmaceutical and biotech industry.
