LAI HBV Therapy – LA NARTI for the treatment of HBV
Speaker: Arnab Chatterjee, Vice President of Medicinal Chemistry at
California Institute for Biomedical Research (Calibr), Scripps Research

Shared progress in the development of long-acting parent entecavir (ETV) and prodrugs for parenteral HBV treatment.
Long-acting treatment for HBV is an unmet need.
- Currently approved oral nucleoside analogue reverse transcriptase inhibitors (NARTIs) carry minimal risk of resistance, achieve >99% viral suppression and are well-tolerated, but require lifelong, daily dosing to achieve and maintain viral suppression.
- Daily adherence is a burden – convenience and access issues make patients susceptible to missed treatment and disease relapse.
- Calibr takes a broad approach to developing LAI medicines – integrated platform between solid-state chemistry and formulation development with in-house molecular and pharmacology resources to allow the team to quickly pivot and optimize a drug candidate (e.g. alter the structure or generate a different solid-state form for IM and SC injection).
Development of LAI ETV for chronic HBV treatment.
- Optimal characteristics from TPP include: q6mo dosing, small dose volume (≤1ml) for self-administered SC injection, low viscosity to enable smaller needle (27G), and low cost for use in LMICs (COGs $100/treatment).
- ETV has reasonable properties as a low-dose oral drug – IC50 0.5nM, low plasma protein binding (13%) and low clearance.
Suspension-based depot strategy for parent ETV (20mpk IM ETV oil suspension).
- ETV is a good LAI candidate – high melting point, solubility in various excipients (oil solubility allows easy passage through 27G needle), and API formulation shows modest clearance and good potency (enables low injection volumes).
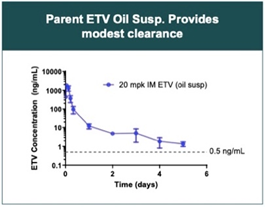
• Observed high peak to trough level with a relatively high Cmax (>1000ng/mL), even with IM oil-based suspension. Would like to lower the Cmax and extend the total AUC to maintain exposure over a prolonged time period (flatten the curve).
Solution-based depot strategy for ETV prodrugs.
- >30 ETV prodrugs synthesized (esters and carbonates) – narrowed candidate selection via solubility measurement, prodrug functionality evaluation (turnover studies in plasma, hepatocytes and microsomes), and follow-up PK modeling in animals.
- Bis-DHA compound (CBR540) provides the most favorable PK profile.
- A single injection of CBR540 oil solution achieved a lower Cmax and extended exposure (above therapeutic for over 1 month) in rats compared to crystalline parent ETV oil suspension.
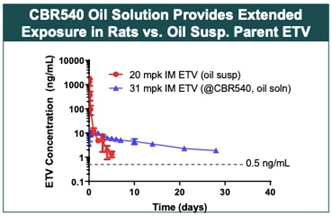
- Production of ETV and intermediate metabolites enable slow release.
- A single injection of CBR540 oil solution achieved a lower Cmax and extended exposure (above therapeutic for over 1 month) in rats compared to crystalline parent ETV oil suspension.
- Mono-DHA compound (CBR261) is likely a good candidate for suspension; many candidates were crystalline (advantageous from GMP perspective).
Summary and Next Steps.
- Generated a series of prodrugs that provide sustained release of ETV after a single IM injection (33-fold improvement in mean residence time).
- CBR540 provides LA depot release of ETV in rats (low injection volumes achieve >150-fold lower Cmax and prolonged exposure relative to oil suspension of parent drug).
- Predicted human dose volume <500 mL for 1-month coverage.
- ISRs were not observed in rats or non-rodents studied (no histology).
- Next steps
- Complete detailed ISR studies and preclinical toxicology studies to better understand the safety of intermediate metabolites.
- Examine role of SC administration for self-administration.
- Perform detailed human dose projections as dose-escalating PK data are generated.
