Focus Group 4 - Challenges in developing combination LA products and regimens
How Well-Matched Should Products Be?
Primary considerations for any combination formulation.
- Same as for any single LA formulation.
- Safety, efficacy and tolerability.
- Target Product Profile (TPP): design and develop with the end goal in mind.
Rank order of Matching options – “simpler is better.”
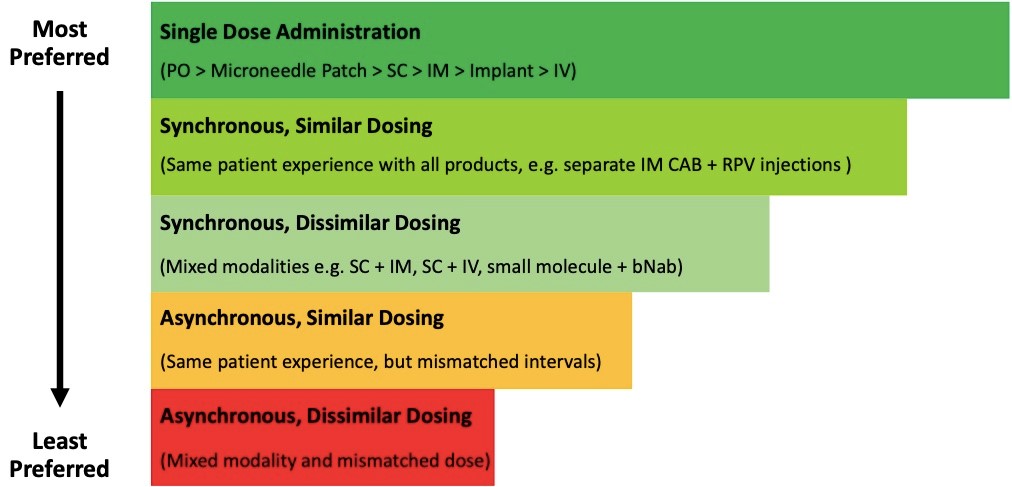
Additional Considerations.
- Longer dosing intervals are appealing, but may introduce trade-offs and less optimal profiles.
-- Require higher drug volumes; low utility for infants and children with rapid changes in drug disposition. - Different populations have different TPPs.
-- Infants and children <2y: rapid metabolic and weight changes; injections are difficult.
-- Pregnancy – PK changes during pregnancy and postpartum.
-- MSM in Brazil prefer injectable PrEP, whereas other populations prefer pills. - Self-administration (pills or patch) is a big advantage vs HCP administration.
- Ready-to-use vs reconstitution and other dose admnistration issues.
Should the inherent characteristics of the API drive development of LA formulations?
- Not all APIs are amenable to combinations.
- Not all APIs will be amenable to all LA formuations.
- To match API to delivery system, either manipulate the drug or manipulate the system.
- Potential for including “boosters” to modify hepatic metabolism of one of the drugs to better align the match between dissimilar APIs and the delivery system.
Combining LA formulations could potentially address multiple use cases.
- HIV PrEP + hormonal contraceptives.
- HIV PrEP + opioid use disorder treatment (e.g., buprenorphine).
- HIV treatment + HBV treatment.
Special considerations for LA combination therapy for HIV in Latin America, Asia and SSA.
- DDIs, particularly TB treatment (rifamycins)
- HBV coinfection.
- Policy, regulatory, and implementation issues can block or delay access to effective treatments.
Mothers and children.
- Infants will require frequent dose adjustments – short dosing intervals and flexible dosing formulations are needed.
- MAP advantages:
-- Avoids injections (challenging in the very young).
-- Potential for dose adjustments for age, weight and gestational age.
-- Self (or caregiver) administration.
-- Can be removed. - bNAb advantages: Multiple delivery options (IM, SC, IV). Maybe easier/safer than small molecules.
- Therapeutic drug monitoring could inform dose adjustments, but will be challenging in terms of access and implementation.
Consensus is that there are differences for prevention vs treatment – examples:
- LA mono-entity efficacy enables better product profiles for PrEP than for treatment.
- LA PrEP has potential for longer dosing intervals.
- Efficacy for PrEP may be achieved with lower drug levels than for treatment.
- Side effect vs efficacy balance is different for PrEP and treatment – PrEP for “healthy” people requires a higher safety profile.
Two vs three drugs?
Safety and efficacy are the priority, not the number of agents.
- May not even need 2-3 agents for some case inidications.
- Enhanced potency and higher barrier to resistance of current ARTs and pipeline, as well as favorable PK of some LA/ER formulations.
Summary of considerations for combinations of LA/ER formulations
Safety and efficacy are the first priorities – after that “simple” is better. Achieving “Simplicity” depends on:
- The ability to match APIs when possible.
- The ability to match delivery systems and dosing interval when possible.
- Addressing the needs of different populations that might require different formulations and have different use cases.
- Recognizing that different formulations will have different market forces and different regulatory, implementation and scalability issues.
- PrEP and treatment use cases will likely require different formulations.
- The Islatravir story is a “wake up call” – plans for combination therapy may have been derailed “late in the game” by unexpected side effects.
